Abstract
Background: The ECLIPSE trial demonstrated that the new film-coated tablet (FCT) deferasirox (DFX) formulation and the dispersible tablet (DT) had similar safety profiles during 6 months of treatment, although fewer patients experienced severe gastrointestinal-related adverse events, and more patients experienced favorable patient-reported outcomes with FCT than with DT (Taher et al. Am J Hematol 2017). Additionally, FCT patients had a greater reduction in serum ferritin (SF) levels from baseline to end of treatment (geometric mean change −431 vs −153 ng/mL in DT patients) despite both groups experiencing similar DFX exposure, which suggests greater efficacy with FCT. In the absence of FCT efficacy data beyond 6 months, we developed a model to predict SF levels in patients from the ECLIPSE trial if they had continued DFX DT or FCT treatment for 6-12 months.
Methods: ECLIPSE was a Phase II, randomized, open-label study in which patients with transfusion-dependent thalassemia (TDT) or International Prognostic Scoring System lower-risk myelodysplastic syndromes (MDS) were randomized to receive DFX DT or FCT. Planned doses in iron chelation therapy (ICT)-naïve patients were DT 20 mg/kg/day or FCT 14 mg/kg/day based on equivalent FCT doses being 30% lower than DT due to improved bioavailability. ICT pre-treated patients received either a DT dose equivalent to their pre-washout dose or a planned 30% reduction for FCT. Dose was adjusted based on SF levels and investigator's judgment every 4 weeks for ICT-naïve patients and every 3 months for ICT pre-treated patients (DT ±5-10 mg/kg/day, max 40 mg/kg/day; FCT ±3.5-7 mg/kg/day, max 28 mg/kg/day); dose adjustments for safety reasons were permitted at any time.
Models for predicting ICT effect were built and tested based on 12-month data from the controlled registration study ICL670A108 (Porter et al. Eur J Haematol 2008). Most patients from ECLIPSE had received prior ICT; therefore, for the development of the model, this situation was mimicked using ICL670A108 month 3-9 data for fitting, and month 10-12 data for evaluating model prediction quality. The selected model accounted for baseline SF levels, assigned daily DFX dose per arm and patients' body weight, and predicted the ICT effect for up to 12 months if patients had continued with their therapy (DT or FCT). The model was applied to both DT and FCT arms of the ECLIPSE trial and predictions were obtained from individual estimates of each patient.
Results:
Trial results: 173 patients were randomized 1:1 to DT (n=86) or FCT (n=87); 70 patients in each arm had TDT and 16 patients had MDS (underlying disease missing in 1 FCT patient). Mean±SD age was 34.9±19.3 years, 51% were female and 90% had previously received ICT. Mean±SD actual daily DFX dose over 24 weeks was 27.5±7.7 mg/kg/day (DT) and 20.8±5.4 mg/kg/day (FCT). At baseline, SF geometric mean was 2381 ng/mL (DT) and 2721 ng/mL (FCT).
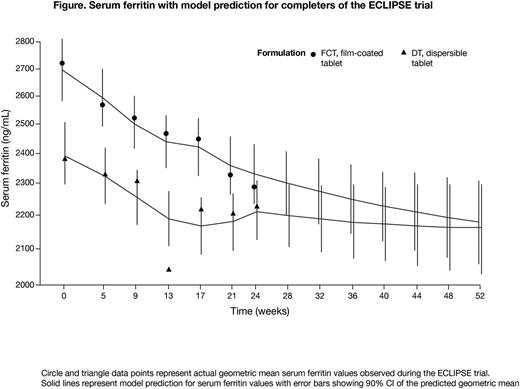
Model prediction: Model diagnostics suggested a close approximation of the ECLIPSE data, as the predicted SF geometric mean during the first 6 months of treatment was similar to the actual SF values observed during the trial (Figure). The model predicted that SF geometric mean values would continue to decrease over the subsequent 6 months with both DT and FCT, reaching 2162 ng/mL (90% confidence interval [CI] 2029-2304) with DT and 2180 ng/mL (90% CI 2054-2311) with FCT after 12 months of treatment. This corresponds to a predicted, relative decrease of 10% with DT and 19% with FCT.
Conclusions: The 6-month results of the model were similar to the actual SF values observed during the ECLIPSE trial, and reliably predicted continued ICT effect for months 7-12 if patients had continued with their assigned treatment (DT or FCT). Previous analyses have indicated that improved patient-reported outcomes, especially increased adherence, are significant mediators of the association between treatment with DT vs FCT and SF reduction from baseline over 6 months of treatment (Taher et al. Haematologica 2017;102[s2]:abst P286). According to the predicted results from this model, and at the doses given in this study, patients treated with FCT would have had a greater reduction in SF at 12 months compared with patients treated with DT; although it should be noted that FCT patients had higher baseline SF. This analysis suggests the FCT offers patients an improved DFX formulation, which could be attributed to improved treatment adherence.
Taher: Novartis Pharmaceuticals: Honoraria, Research Funding; Celgene: Research Funding. Weber: Novartis: Employment. Han: Novartis: Employment. Bruederle: Novartis: Employment. Porter: Celegene: Consultancy; Agios Pharmaceuticals: Consultancy, Honoraria; Shire: Consultancy, Honoraria; Bluebird Bio: Consultancy; Novartis: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal